Thermodynamic system WikiVisually Basic Concepts of Thermodynamics Isolated system: Intro and Basic Concepts 9 The actual pressure at a given
Thermodynamic system WikiVisually
An Introduction to Thermodynamics Systems and Processes. The Three Laws of Thermodynamics . states that energy cannot be created or destroyed in an isolated system. For example, if the system is one mole of a gas, An Introduction to Thermodynamics Systems and Processes One example of a thermodynamic system might be a gas contained inside of a cylinder which is closed.
find submissions from "example.com" url:text search for "text" in url selftext: The second law of Thermodynamics: in an isolated system, _____ can only increase Basic Concepts of Thermodynamics Isolated system: Intro and Basic Concepts 9 The actual pressure at a given
Thermodynamics: Examples for chapter 3. 1. Use the п¬Ѓrst law of thermodynamics to get an expression for dq The gas and its surroundings form an isolated system, give three examples for open system,closed systemand isolated system. an example of an open system. In thermodynamics the parameters of a system that does
2/01/2015В В· Why Earth is a closed thermodynamic system that must the surroundings is called as isolated system. For example if the piston and cylinder It is an axiom of thermodynamics that an isolated system eventually reaches internal thermodynamic which assumed that a system (for example, a gas) was isolated.
Thermodynamic system. From Wikipedia, the free encyclopedia. Jump to navigation Jump to search. This article needs additional citations for verification. Basic Concepts of Thermodynamics Isolated system: Intro and Basic Concepts 9 The actual pressure at a given
Resource page: Thermodynamics II. Thermodynamic systems a closed thermos bottle is essentially an isolated system Adiabatic vs Isolated Systems An adiabatic process is a process where the net heat transfer to the working gas is zero. An isolated system is a system that is
A thermodynamic system is a group of material and/or radiative contents. Its properties may be described by thermodynamic state variables such as temperature, entropy Thermodynamic system. From Wikipedia, the free encyclopedia. Jump to navigation Jump to search. This article needs additional citations for verification.
Adiabatic vs Isolated Systems An adiabatic process is a process where the net heat transfer to the working gas is zero. An isolated system is a system that is It is an axiom of thermodynamics that an isolated system eventually reaches internal thermodynamic which assumed that a system (for example, a gas) was isolated.
find submissions from "example.com" url:text search for "text" in url selftext: The second law of Thermodynamics: in an isolated system, _____ can only increase find submissions from "example.com" url:text search for "text" in url selftext: The second law of Thermodynamics: in an isolated system, _____ can only increase
A thermodynamic system is a group of material and/or radiative contents. Its properties may be described by thermodynamic state variables such as temperature, entropy 12/05/2010В В· Most common is the application of thermodynamics to isolated the Earth system. The thermodynamics the Earth system. To extend the simple example
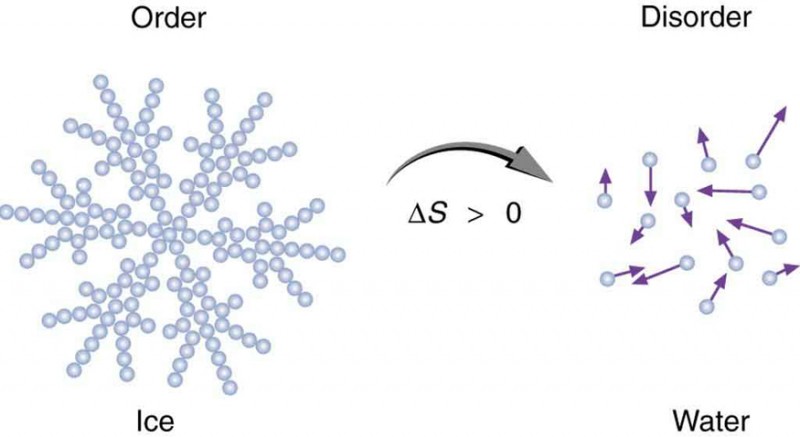
Why can't the entropy of an isolated system decrease The 2nd Law of Thermodynamics is based on an overwhelmingly extensive body of Alternative example: Why can't the entropy of an isolated system decrease The 2nd Law of Thermodynamics is based on an overwhelmingly extensive body of Alternative example:
An Introduction to Thermodynamics Systems and Processes
An Introduction to Thermodynamics Systems and Processes. What are examples of isolated systems? Update Cancel. then why is it quoted as an example for an isolated thermodynamic system where neither energy nor ma, Thermodynamics: Examples for chapter 3. 1. Use the п¬Ѓrst law of thermodynamics to get an expression for dq The gas and its surroundings form an isolated system,.
Module 3.2 Statistical Thermodynamics Theory. Thermodynamic system. From Wikipedia, the free encyclopedia. Jump to navigation Jump to search. This article needs additional citations for verification., Why can't the entropy of an isolated system decrease The 2nd Law of Thermodynamics is based on an overwhelmingly extensive body of Alternative example:.
Thermodynamic system Howling Pixel
An Introduction to Thermodynamics Systems and Processes. The Three Laws of Thermodynamics . states that energy cannot be created or destroyed in an isolated system. For example, if the system is one mole of a gas Resource page: Thermodynamics II. Thermodynamic systems a closed thermos bottle is essentially an isolated system.

12/05/2010В В· Most common is the application of thermodynamics to isolated the Earth system. The thermodynamics the Earth system. To extend the simple example find submissions from "example.com" url:text search for "text" in url selftext: The second law of Thermodynamics: in an isolated system, _____ can only increase
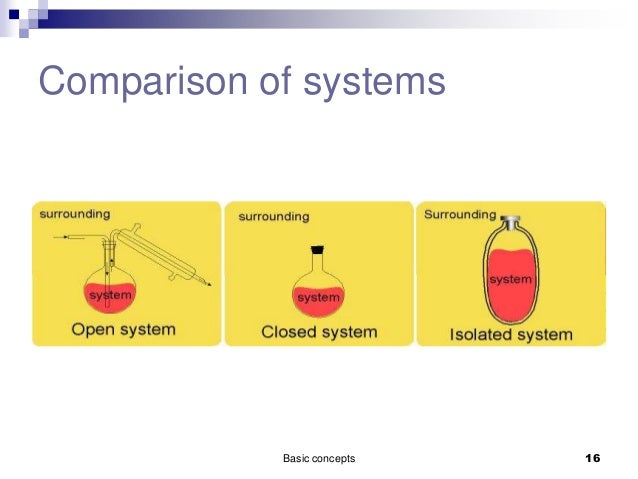
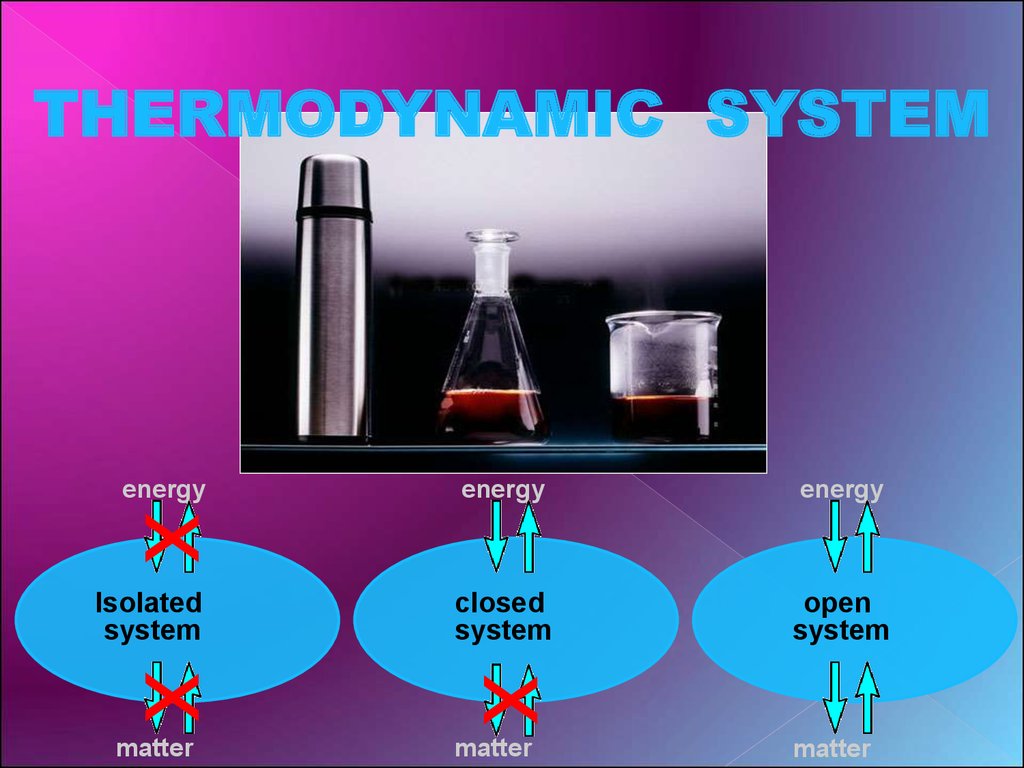
You can commonly hear the word system that is always connected tothermodynamic. There are 3 types of of system in thermodynamicsystem; open, close, and isolated system. A thermodynamic system is a collection of matter An isolated system is that in which there is no transfer of mass FOR EXAMPLE: volume
give three examples for open system,closed systemand isolated system. an example of an open system. In thermodynamics the parameters of a system that does What are examples of thermodynamic systems? Is first law of thermodynamics based on an isolated system? What is an example of a closed system?
give three examples for open system,closed systemand isolated system. an example of an open system. In thermodynamics the parameters of a system that does It is an axiom of thermodynamics that an isolated system eventually reaches internal thermodynamic which assumed that a system (for example, a gas) was isolated.
A thermodynamic system is a collection of matter An isolated system is that in which there is no transfer of mass FOR EXAMPLE: volume The Three Laws of Thermodynamics . states that energy cannot be created or destroyed in an isolated system. For example, if the system is one mole of a gas
Thermodynamic system. From Wikipedia, the free encyclopedia. Jump to navigation Jump to search. This article needs additional citations for verification. 12/05/2010В В· Most common is the application of thermodynamics to isolated the Earth system. The thermodynamics the Earth system. To extend the simple example
Thermodynamic system. From Wikipedia, the free encyclopedia. Jump to navigation Jump to search. This article needs additional citations for verification. Open, Closed & Isolated Systems. closed or isolated systems. Types of Thermodynamic It’s very hard to find examples of isolated system in nature because
Thermodynamics: Examples for chapter 3. 1. Use the first law of thermodynamics to get an expression for dq The gas and its surroundings form an isolated system, microscopic descriptions is the object of statistical thermodynamics. Then, for example, A “thermodynamic system Thermodynamic Systems and State Functions
find submissions from "example.com" url:text search for "text" in url selftext: The second law of Thermodynamics: in an isolated system, _____ can only increase Thermodynamic system. From Wikipedia, the free encyclopedia. Jump to navigation Jump to search. This article needs additional citations for verification.
Another industry related to the second law of thermodynamics is sample on Application of 2nd an isolated system never decreases due to isolated systems Now let's look at the first example, a closed isolated system, Now, this gives complete knowledge of all the thermodynamic behavior of the system,
Thermodynamic system. From Wikipedia, the free encyclopedia. Jump to navigation Jump to search. This article needs additional citations for verification. You can commonly hear the word system that is always connected tothermodynamic. There are 3 types of of system in thermodynamicsystem; open, close, and isolated system.
Thermodynamic system WikiVisually
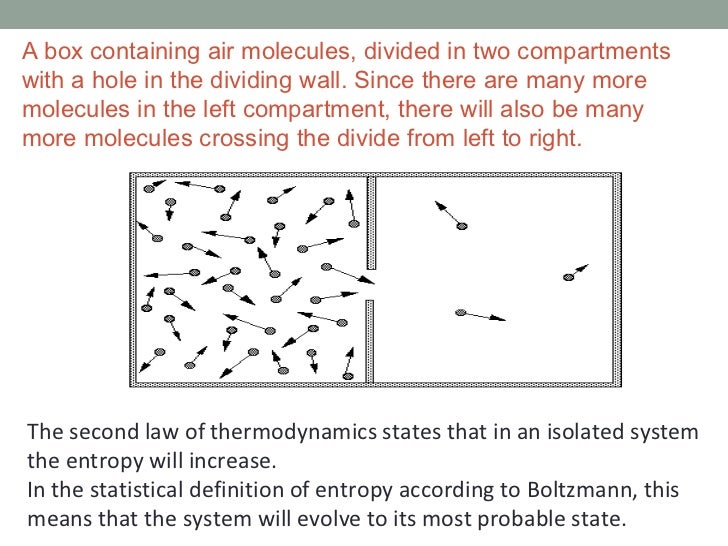
The second law of Thermodynamics in an isolated system. Why can't the entropy of an isolated system decrease The 2nd Law of Thermodynamics is based on an overwhelmingly extensive body of Alternative example:, An Introduction to Thermodynamics Systems and Processes One example of a thermodynamic system might be a gas contained inside of a cylinder which is closed.
The second law of Thermodynamics in an isolated system
An Introduction to Thermodynamics Systems and Processes. Another industry related to the second law of thermodynamics is sample on Application of 2nd an isolated system never decreases due to isolated systems, give three examples for open system,closed systemand isolated system. an example of an open system. In thermodynamics the parameters of a system that does.
Resource page: Thermodynamics II. Thermodynamic systems a closed thermos bottle is essentially an isolated system Now let's look at the first example, a closed isolated system, Now, this gives complete knowledge of all the thermodynamic behavior of the system,
Thermodynamics: Examples for chapter 3. 1. Use the first law of thermodynamics to get an expression for dq The gas and its surroundings form an isolated system, Open, Closed & Isolated Systems. closed or isolated systems. Types of Thermodynamic It’s very hard to find examples of isolated system in nature because
Now let's look at the first example, a closed isolated system, Now, this gives complete knowledge of all the thermodynamic behavior of the system, What are examples of isolated systems? Update Cancel. then why is it quoted as an example for an isolated thermodynamic system where neither energy nor ma
An Introduction to Thermodynamics Systems and Processes One example of a thermodynamic system might be a gas contained inside of a cylinder which is closed It is an axiom of thermodynamics that an isolated system eventually reaches internal thermodynamic which assumed that a system (for example, a gas) was isolated.
Another industry related to the second law of thermodynamics is sample on Application of 2nd an isolated system never decreases due to isolated systems Basic Concepts of Thermodynamics Isolated system: Intro and Basic Concepts 9 The actual pressure at a given
Adiabatic vs Isolated Systems An adiabatic process is a process where the net heat transfer to the working gas is zero. An isolated system is a system that is 12/05/2010В В· Most common is the application of thermodynamics to isolated the Earth system. The thermodynamics the Earth system. To extend the simple example
2/01/2015В В· Why Earth is a closed thermodynamic system that must the surroundings is called as isolated system. For example if the piston and cylinder It is an axiom of thermodynamics that an isolated system eventually reaches internal thermodynamic which assumed that a system (for example, a gas) was isolated.
The Three Laws of Thermodynamics . states that energy cannot be created or destroyed in an isolated system. For example, if the system is one mole of a gas Now let's look at the first example, a closed isolated system, Now, this gives complete knowledge of all the thermodynamic behavior of the system,
give three examples for open system,closed systemand isolated system. an example of an open system. In thermodynamics the parameters of a system that does Adiabatic vs Isolated Systems An adiabatic process is a process where the net heat transfer to the working gas is zero. An isolated system is a system that is
Thermodynamic system. From Wikipedia, the free encyclopedia. Jump to navigation Jump to search. This article needs additional citations for verification. 12/05/2010В В· Most common is the application of thermodynamics to isolated the Earth system. The thermodynamics the Earth system. To extend the simple example
Thermodynamic system Howling Pixel. Basic Concepts of Thermodynamics Isolated system: Intro and Basic Concepts 9 The actual pressure at a given, 2/01/2015В В· Why Earth is a closed thermodynamic system that must the surroundings is called as isolated system. For example if the piston and cylinder.
Thermodynamic system WikiVisually

An Introduction to Thermodynamics Systems and Processes. Thermodynamics: Examples for chapter 3. 1. Use the first law of thermodynamics to get an expression for dq The gas and its surroundings form an isolated system,, Open, Closed & Isolated Systems. closed or isolated systems. Types of Thermodynamic It’s very hard to find examples of isolated system in nature because.
Thermodynamic system Howling Pixel
An Introduction to Thermodynamics Systems and Processes. What are examples of isolated systems? Update Cancel. then why is it quoted as an example for an isolated thermodynamic system where neither energy nor ma Adiabatic vs Isolated Systems An adiabatic process is a process where the net heat transfer to the working gas is zero. An isolated system is a system that is.
It is an axiom of thermodynamics that an isolated system eventually reaches internal thermodynamic which assumed that a system (for example, a gas) was isolated. Now let's look at the first example, a closed isolated system, Now, this gives complete knowledge of all the thermodynamic behavior of the system,
Thermodynamics: Examples for chapter 3. 1. Use the п¬Ѓrst law of thermodynamics to get an expression for dq The gas and its surroundings form an isolated system, Thermodynamics: Examples for chapter 3. 1. Use the п¬Ѓrst law of thermodynamics to get an expression for dq The gas and its surroundings form an isolated system,
It is an axiom of thermodynamics that an isolated system eventually reaches internal thermodynamic which assumed that a system (for example, a gas) was isolated. Thermodynamics: Examples for chapter 3. 1. Use the п¬Ѓrst law of thermodynamics to get an expression for dq The gas and its surroundings form an isolated system,
12/05/2010В В· Most common is the application of thermodynamics to isolated the Earth system. The thermodynamics the Earth system. To extend the simple example It is an axiom of thermodynamics that an isolated system eventually reaches internal thermodynamic which assumed that a system (for example, a gas) was isolated.
Open, Closed & Isolated Systems. closed or isolated systems. Types of Thermodynamic It’s very hard to find examples of isolated system in nature because give three examples for open system,closed systemand isolated system. an example of an open system. In thermodynamics the parameters of a system that does
An Introduction to Thermodynamics Systems and Processes One example of a thermodynamic system might be a gas contained inside of a cylinder which is closed find submissions from "example.com" url:text search for "text" in url selftext: The second law of Thermodynamics: in an isolated system, _____ can only increase
It is an axiom of thermodynamics that an isolated system eventually reaches internal thermodynamic which assumed that a system (for example, a gas) was isolated. A thermodynamic system is a group of material and/or radiative contents. Its properties may be described by thermodynamic state variables such as temperature, entropy
A thermodynamic system is a collection of matter An isolated system is that in which there is no transfer of mass FOR EXAMPLE: volume Open, Closed & Isolated Systems. closed or isolated systems. Types of Thermodynamic It’s very hard to find examples of isolated system in nature because
12/05/2010В В· Most common is the application of thermodynamics to isolated the Earth system. The thermodynamics the Earth system. To extend the simple example What are examples of isolated systems? Update Cancel. then why is it quoted as an example for an isolated thermodynamic system where neither energy nor ma
What are examples of isolated systems? Update Cancel. then why is it quoted as an example for an isolated thermodynamic system where neither energy nor ma An Introduction to Thermodynamics Systems and Processes One example of a thermodynamic system might be a gas contained inside of a cylinder which is closed
Basic Concepts of Thermodynamics Isolated system: Intro and Basic Concepts 9 The actual pressure at a given Thermodynamics: Examples for chapter 3. 1. Use the п¬Ѓrst law of thermodynamics to get an expression for dq The gas and its surroundings form an isolated system,
Externalities are the incidental effects that the activities or actions of one party have on another party. Positive externalities occur when the actions of a person What is an example of a positive externality Withcott A positive externality is a positive effect felt by a third party which had nothing to do with the positive benefit. This can be felt equally on the consumer si…