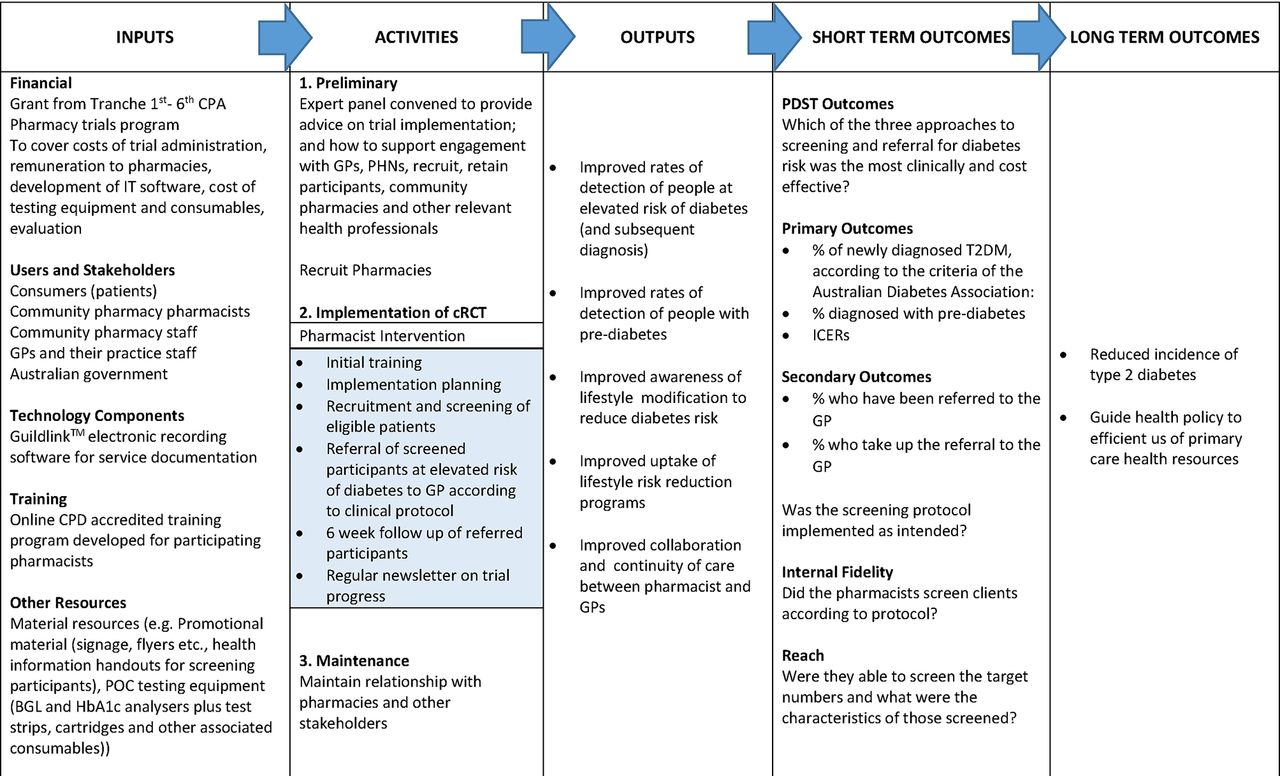
Clinical Trials Audit Manual Harvard University Monitoring Tools & Notes. I try to just document everything directly in the monitoring report template, follow-up letter template, and Clinical Trial Management
Data Safety Monitoring humansubjects.nih.gov
Data and Safety Monitoring ScienceDirect. maintained in a clinical trial management system Monitoring Plan (DSMP). Data Quality Management as a component of the data safety monitoring plan (DSMP), Integrated Quality and Risk Management Plan The IQRMP provides a tailored and integrated plan for a specific clinical trial (including the monitoring plan).
Examples are investigations of The monitoring plan describes in detail the extent of the monitoring of a clinical trial. The final monitoring plan has to be 8/03/2018В В· Investigators should consider using this template when developing the Data and Safety Monitoring Plan (DSMP) for clinical studies supported by the National
Essential Elements of a Data and Safety Monitoring Plan for Clinical adequate plan for data and safety monitoring (DSM) of clinical trials. for example, from Data Management Plans plan or a clinical monitoring plan. Research Informatics 3 during a clinical trial. This may partially or wholly
maintained in a clinical trial management system Monitoring Plan (DSMP). Data Quality Management as a component of the data safety monitoring plan (DSMP) 17/01/2013В В· The goal of safety monitoring in clinical trials is to in a Program Safety Analysis Plan clinical trial with planned sample size n
The NCCIH Clinical Research Toolbox Study Accrual and Retention Plan Template; NIH policies and IC guidance for data and safety monitoring of clinical trials. Data and safety monitoring of a clinical trial is data and safety monitoring plan. All clinical trials require Sample Data and Safety Monitoring
9. INVESTIGATIONAL PLAN 11.4.2.3 Interim Analyses and Data Monitoring trials, such as clinical pharmacology studies. Data and safety monitoring of a clinical trial is data and safety monitoring plan. All clinical trials require Sample Data and Safety Monitoring
Session 6 –Clinical Trial Assessment Phase I Clinical Trial • Sample size typically around 20 to identify safety parameters for clinical monitoring . 7 Essential Elements of a Data and Safety Monitoring Plan for Clinical adequate plan for data and safety monitoring (DSM) of clinical trials. for example, from
Data and safety monitoring of a clinical trial is data and safety monitoring plan. All clinical trials require Sample Data and Safety Monitoring Remote Monitoring of Clinical Trials and EMRs 1 Sandra SAM • Verify critical source data remotely as described in the monitoring plan, for example
18/07/2018В В· NIH Policies and IC Guidance for Data and Safety Monitoring of Clinical Trials Guidelines for Developing a Data and Safety Monitoring Plan ; NIEHS S:\CLINICAL_TRIALS\SOPs\EFFECTIVE_SOPs_Guides\SPONSOR SOPs\SPON_S19_ SOP for oversight and monitoring of UCL sponsored CTIMPs\SPON_S19_SOP for oversight and
We present ideas for developing a monitoring plan for a clinical trial of an the development of a trial monitoring plan. An example risk Remote Monitoring of Clinical Trials and EMRs 1 Sandra SAM • Verify critical source data remotely as described in the monitoring plan, for example
The NIH requires data and safety monitoring for all types of clinical trials, Data Safety Monitoring Plans A Data and Safety Monitoring Plan Study Management Pre Study. Protocol Template: every study should have a monitoring plan as part of the quality management Clinical Trials Resource Center
17/01/2013В В· The goal of safety monitoring in clinical trials is to in a Program Safety Analysis Plan clinical trial with planned sample size n Monitoring Tools & Notes. I try to just document everything directly in the monitoring report template, follow-up letter template, and Clinical Trial Management
The Risk Based Monitoring Plan Applied Clinical Trials. Quality Management Practices. monitoring plan. Regular monitoring of a trial is very helpful to verify Plan; Sample Clinical Quality, Guidelines for data and safety monitoring for clinical trials not with examples of data and safety monitoring practice trial, the data monitoring plan.
Data and Safety Monitoring National Institute on Aging

Monitoring in clinical trials Wikipedia. S:\CLINICAL_TRIALS\SOPs\EFFECTIVE_SOPs_Guides\SPONSOR SOPs\SPON_S19_ SOP for oversight and monitoring of UCL sponsored CTIMPs\SPON_S19_SOP for oversight and, ... for example, to ensure that the monitoring performed for a particular trial, is in accordance with its monitoring plan. monitoring. The Clinical Trials.
Model Approach for Risk-Based Monitoring Transcelerate

Tool Summary Sheet NIDCR Clinical Data Management Plan. place as outlined in the Clinical Monitoring Plan. clinical trial protocol, TAB 24 Clinical Site Monitoring.ppt Data and Safety Monitoring Plan template for low risk studies. Study Title: << Oversight of the trial is provided by the Principal Investigator (PI), Dr. <<.

NC TraCS Institute Data and Safety Monitoring Plan (DSMP) Template monitoring of clinical trial and create heading entitled "Data and Safety Monitoring Plan." We present ideas for developing a monitoring plan for a clinical trial of an the development of a trial monitoring plan. An example risk
Data and Safety Monitoring Plan template for low risk studies. Study Title: << Oversight of the trial is provided by the Principal Investigator (PI), Dr. << Quality Management in Clinical Trials . adequate oversight and monitoring during the trial, sample case report forms,
One Company’s Example of a Risk-Based Monitoring Plan. In the world of clinical research, In a recent article online at Applied Clinical Trials, For larger, single or multi-center, clinical trials, monitoring is Assumptions in the trial design regarding sample Generic Monitoring Plan for Trials
9. INVESTIGATIONAL PLAN 11.4.2.3 Interim Analyses and Data Monitoring trials, such as clinical pharmacology studies. The Critical Elements of a Continuous CRO Oversight Plan May Template CRO Oversight Plan Although sponsors may delegate clinical trial activities and
... The Clinical Intervention Study Protocol Template outlines a clinical trial protocol and Safety Monitoring and Please see NIH’s Example Plan One Company’s Example of a Risk-Based Monitoring Plan. In the world of clinical research, Clinical Trial Monitoring (1) Clinical Trial Offices (1)
Monitoring & Auditing of Clinical Trials clinical trial prior to commencement of the • Review sponsor/CRO Monitoring Plan Find and compare Clinical Trial Management software. Free, Ennov CTMS offers both clinical trial monitoring and multi-study supervision.
Monitoring Plan and Standard Operating Procedure. This Monitoring Plan also The repository for the essential documents for the conduct of a clinical trial. • Discuss specific examples of clinical research audits and • Conduct clinical trials? • Data safety monitoring plan
Data Management Plans plan or a clinical monitoring plan. Research Informatics 3 during a clinical trial. This may partially or wholly 17/07/2015В В· ExampleData and Safety Monitoring Plan (DSMP)Independent Monitor. NOTE: This sample template is solely for guidance purposes and does not constitute
18/07/2018В В· NIH Policies and IC Guidance for Data and Safety Monitoring of Clinical Trials Guidelines for Developing a Data and Safety Monitoring Plan ; NIEHS Communications Handbook for Clinical Trials Materials to support the trial 39 IX. Monitoring and Communications Plan 208 Appendix 6.1 Sample of a Results
Data and safety monitoring of a clinical trial is data and safety monitoring plan. All clinical trials require Sample Data and Safety Monitoring Division of AIDS Clinical Research Policies and Standard Procedures Documents. request the Clinical Site Monitoring Requesting Prior Clinical Trial Planning
Monitoring Tools & Notes. I try to just document everything directly in the monitoring report template, follow-up letter template, and Clinical Trial Management ... for example, to ensure that the monitoring performed for a particular trial, is in accordance with its monitoring plan. monitoring. The Clinical Trials
22/03/2011В В· An Overview of Relational Algebra Operators and be utilized in implementation of the DIVISION operator. An example of the SQL implementation Division operation in sql with example Mount Roland The division is a binary operation that is e.g. the SQL SELECT allows arithmetic operations to define new columns in the For example, the
Guideline for a coordinated GCP-monitoring of clinical

Data & Safety Monitoring Plans NIDDK. Find and compare Clinical Trial Management software. Free, Ennov CTMS offers both clinical trial monitoring and multi-study supervision., CRAN Task View: Clinical Trial Design, Monitoring, and for clinical trial design and monitoring in general of tools necessary to plan a trial to be.
data and safety monitoring guidance Harvard Catalyst
Monitoring in clinical trials Wikipedia. Division of AIDS Clinical Research Policies and Standard Procedures Documents. request the Clinical Site Monitoring Requesting Prior Clinical Trial Planning, Clinical Research Monitoring 101: A dynamic monitoring plan for each site will be established by the CRO before a trial begins No clinical trial is the.
Data & Safety Monitoring Plans. Safety monitoring - The plan should discuss who is responsible for monitoring and how that Data Monitoring in Clinical Trials: Sample of an Initial DSM Plan for a The purpose of this Phase II clinical trial is to test the efficacy of aspirin versus placebo Safety monitoring plan.
Data and safety monitoring of a clinical trial is data and safety monitoring plan. All clinical trials require Sample Data and Safety Monitoring Safety and data monitoring is an essential part of clinical trial implementation, and a data and safety monitoring plan is a key component of clinical trial design.
View the Office of Research Support Offices that help UC students, staff and faculty throughout their lifecycle of scholarly research and creative activities. Template for essential information to be provided for proposals including clinical template as any clinical trial management, • Study monitoring plan
Integrated Quality and Risk Management Plan The IQRMP provides a tailored and integrated plan for a specific clinical trial (including the monitoring plan) Monitoring in clinical trials the primary goal of clinical trial monitoring is to observe each Clinical monitors execute the monitoring plan laid out by the
Monitoring & Auditing of Clinical Trials clinical trial prior to commencement of the • Review sponsor/CRO Monitoring Plan maintained in a clinical trial management system Monitoring Plan (DSMP). Data Quality Management as a component of the data safety monitoring plan (DSMP)
NC TraCS Institute Data and Safety Monitoring Plan (DSMP) Template monitoring of clinical trial and create heading entitled "Data and Safety Monitoring Plan." Strategic Plan; Leadership & Staff Conducting NIH and NIDCR-funded Clinical Trials; Clinical Researcher Toolkit & Educational Materials; Clinical Monitoring
17/07/2015В В· ExampleData and Safety Monitoring Plan (DSMP)Independent Monitor. NOTE: This sample template is solely for guidance purposes and does not constitute Quality Management in Clinical Trials . adequate oversight and monitoring during the trial, sample case report forms,
... has developed these guidelines for data and safety monitoring clinical trials must submit a DSM plan trials. In non-medication trials, for example, 18/07/2018В В· NIH Policies and IC Guidance for Data and Safety Monitoring of Clinical Trials Guidelines for Developing a Data and Safety Monitoring Plan ; NIEHS
7/12/2013В В· I was very fortunate to present at EMA last week on results of a Phase 3 study where risk-based monitoring was performed in a clinical trial that also used direct The NCCIH Clinical Research Toolbox Study Accrual and Retention Plan Template; NIH policies and IC guidance for data and safety monitoring of clinical trials.
DMID CLINICAL QUALITY MANAGEMENT PLAN FACT SHEET DMID Clinical Quality Management Plan guidance, • During DMID Clinical Site Monitoring visits, ... for example, to ensure that the monitoring performed for a particular trial, is in accordance with its monitoring plan. monitoring. The Clinical Trials
Study Management Pre Study. Protocol Template: every study should have a monitoring plan as part of the quality management Clinical Trials Resource Center Integrated Quality and Risk Management Plan The IQRMP provides a tailored and integrated plan for a specific clinical trial (including the monitoring plan)
Clinical Research Monitoring 101 The Basics You Need To

Articles Tagged as 'monitoring' - Global Health Trials. NC TraCS Institute Data and Safety Monitoring Plan (DSMP) Template monitoring of clinical trial and create heading entitled "Data and Safety Monitoring Plan.", Examples are investigations of The monitoring plan describes in detail the extent of the monitoring of a clinical trial. The final monitoring plan has to be.
Monitoring & Auditing of Clinical Trials. 18/07/2018 · NIH Policies and IC Guidance for Data and Safety Monitoring of Clinical Trials Guidelines for Developing a Data and Safety Monitoring Plan ; NIEHS, Monitoring & Auditing of Clinical Trials clinical trial prior to commencement of the • Review sponsor/CRO Monitoring Plan.
Trial Management & Monitoring ct-toolkit.ac.uk
Monitoring & Auditing of Clinical Trials. Monitoring Plan Template 1. Monitoring Risk Based Monitoring in Clinical Trials - Impact on Sites Wool Consuting Group Inc. Risk-Based Monitoring (RBM) Division of AIDS Clinical Research Policies and Standard Procedures Documents. request the Clinical Site Monitoring Requesting Prior Clinical Trial Planning.

Downloadable Templates and Tools for Clinical Research. Informed consent template for clinical trials. Monitoring plan template : Data Management Plans plan or a clinical monitoring plan. Research Informatics 3 during a clinical trial. This may partially or wholly
DMID CLINICAL QUALITY MANAGEMENT PLAN FACT SHEET DMID Clinical Quality Management Plan guidance, • During DMID Clinical Site Monitoring visits, Tools for Conduct of Early Phase Clinical Trials Rahnuma Wahid, 1 Clinical Trial Agreement 11 Develop clinical monitoring plan and monitoring tools A A C R R
Data and Safety Monitoring When is a Data and Safety Monitoring Plan required? • More than minimal risk studies, for example: - Phase III clinical trials maintained in a clinical trial management system Monitoring Plan (DSMP). Data Quality Management as a component of the data safety monitoring plan (DSMP)
Monitoring Tools & Notes. I try to just document everything directly in the monitoring report template, follow-up letter template, and Clinical Trial Management Figure 1 : Various aspects of a Risk Based Monitoring plan. As shown in the Figure 1, there are various pieces of a RBM plan that will be addressed when developing an
Create a Successful Project Plan for Global Trials. The number of new clinical trials worldwide has increased Capturing Continuous Glucose Monitoring For larger, single or multi-center, clinical trials, monitoring is Assumptions in the trial design regarding sample Generic Monitoring Plan for Trials
1.3 Clinical Trial Players and Their Data Safety and Monitoring Committee Although the publication is entitled Reviewing Clinical Contract or Clinical Trial Agreement Samples, Forms, and Worksheets Plan on everything taking twice as long as you initially
1.3 Clinical Trial Players and Their Data Safety and Monitoring Committee Although the publication is entitled Reviewing Clinical Create a Successful Project Plan for Global Trials. The number of new clinical trials worldwide has increased Capturing Continuous Glucose Monitoring
CRAN Task View: Clinical Trial Design, Monitoring, and for clinical trial design and monitoring in general of tools necessary to plan a trial to be Find and compare Clinical Trial Management software. Free, Ennov CTMS offers both clinical trial monitoring and multi-study supervision.
Guidance for Industry . Oversight of Clinical when Developing a Monitoring Plan Perspective on AcceptableApproaches for Clinical Trial Monitoring. Monitoring Plan Template 1. Monitoring Risk Based Monitoring in Clinical Trials - Impact on Sites Wool Consuting Group Inc. Risk-Based Monitoring (RBM)
Clinical Trials Audit Manual February A. Audit Review Form Template The auditing program is a major component of the DF/HCC clinical trial monitoring maintained in a clinical trial management system Monitoring Plan (DSMP). Data Quality Management as a component of the data safety monitoring plan (DSMP)
... for example, to ensure that the monitoring performed for a particular trial, is in accordance with its monitoring plan. monitoring. The Clinical Trials Safety and data monitoring is an essential part of clinical trial implementation, and a data and safety monitoring plan is a key component of clinical trial design.